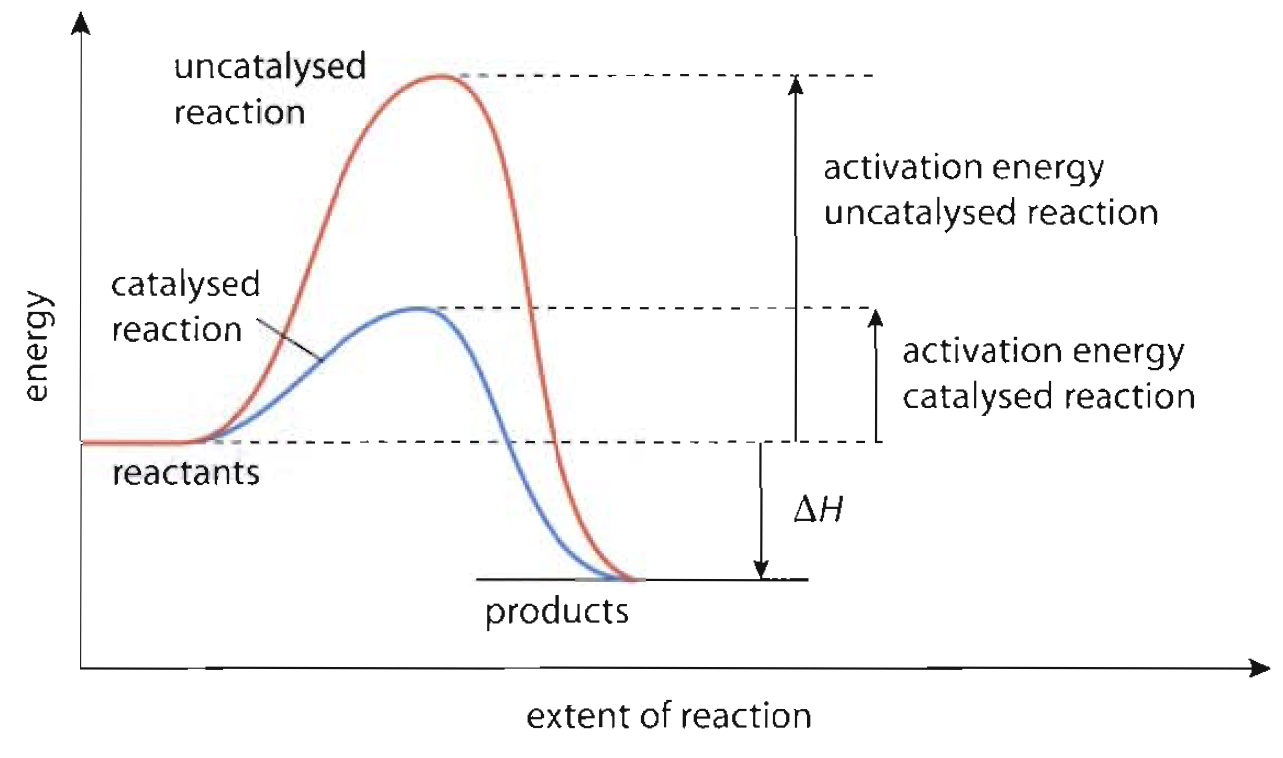
Catalyst Reaction Meaning . A catalyst is a chemical substance. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. In chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. This change is brought about by a specific interaction. Catalysis is the process of speeding up a. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst.
from sheetalschemblog.blogspot.com.tr
In chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. A catalyst is a chemical substance. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. This change is brought about by a specific interaction. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Catalysis is the process of speeding up a. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
Sheetal's Chemistry Blog
Catalyst Reaction Meaning Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysis is the process of speeding up a. A catalyst is a chemical substance. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. This change is brought about by a specific interaction.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst Reaction Meaning A catalyst is a chemical substance. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts function by allowing the reaction to take place through an alternative mechanism that. Catalyst Reaction Meaning.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalyst Reaction Meaning Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance. Catalysis is the process of speeding up a. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysis is the process that alters the rate. Catalyst Reaction Meaning.
From www.pinterest.com
Meaning of catalyst Reasoning Test, English Language Learning, Chemical Catalyst Reaction Meaning In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. This change is brought about by a specific interaction. In chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. Catalysts function by allowing the reaction to take place. Catalyst Reaction Meaning.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of Catalyst Reaction Meaning In chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Catalysis is the process of speeding up a. This change is brought about by a specific interaction. In chemistry and biology,. Catalyst Reaction Meaning.
From sheetalschemblog.blogspot.com.tr
Sheetal's Chemistry Blog Catalyst Reaction Meaning In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Catalysis is the process of speeding up a. This change is brought about by a specific interaction. Catalyst, in. Catalyst Reaction Meaning.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Catalyst Reaction Meaning This change is brought about by a specific interaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Catalysis is the process that alters the rate of a chemical reaction under the influence of. Catalyst Reaction Meaning.
From brainly.in
define catalyst with example ?? Brainly.in Catalyst Reaction Meaning In chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysts. Catalyst Reaction Meaning.
From www.youtube.com
What Are Catalysts? Reactions Chemistry FuseSchool YouTube Catalyst Reaction Meaning This change is brought about by a specific interaction. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. A catalyst is a chemical substance.. Catalyst Reaction Meaning.
From www.slideserve.com
PPT RATES OF REACTION A guide for GCSE students PowerPoint Catalyst Reaction Meaning Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. This change is brought about by a specific interaction. Catalysis is the process of speeding up a. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In chemistry and biology, a catalyst is a. Catalyst Reaction Meaning.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalyst Reaction Meaning In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. This change is brought about by a specific interaction. In chemistry, catalysts are defined as those substances which alter the. Catalyst Reaction Meaning.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalyst Reaction Meaning In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. This change is brought about by a specific interaction. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalyst, in chemistry, any substance that. Catalyst Reaction Meaning.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalyst Reaction Meaning Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without. Catalyst Reaction Meaning.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.9.3 MaxwellBoltzmann Distributions翰林国际教育 Catalyst Reaction Meaning Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysis is the process that alters the rate of a chemical reaction under the influence. Catalyst Reaction Meaning.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst Reaction Meaning Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. A catalyst is a chemical substance. Catalysis is the process of speeding up a. In chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. In chemistry and biology, a catalyst is. Catalyst Reaction Meaning.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation ID591293 Catalyst Reaction Meaning A catalyst is a chemical substance. Catalysis is the process that alters the rate of a chemical reaction under the influence of a catalyst. Catalysis is the process of speeding up a. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. Catalysts function by allowing the. Catalyst Reaction Meaning.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalyst Reaction Meaning A catalyst is a chemical substance. Catalysis, the modification of the rate of a chemical reaction, usually an acceleration, by addition of a substance not consumed during the reaction. This change is brought about by a specific interaction. In chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. In chemistry. Catalyst Reaction Meaning.
From www.slideserve.com
PPT 23.5 Features of homogeneous catalysis PowerPoint Presentation Catalyst Reaction Meaning Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. In chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of. Catalyst Reaction Meaning.
From 2012books.lardbucket.org
Catalysis Catalyst Reaction Meaning Catalysis is the process of speeding up a. Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is the process that alters the rate of a chemical reaction under the influence of a. Catalyst Reaction Meaning.